Figaro offers a wide range of gas sensor products for the detection of various gases, from explosive gases such as propane, toxic gases such as carbon monoxide, to air quality sensors for volatile organic compounds (VOCs) that are responsible for sick-house syndrome. Figaro offers a diverse portfolio of sensor technologies that can be matched to the unique requirements of each application.
Summary
STEP1
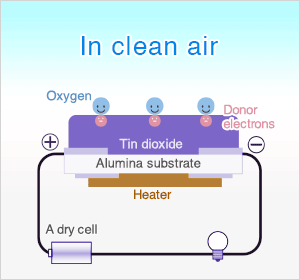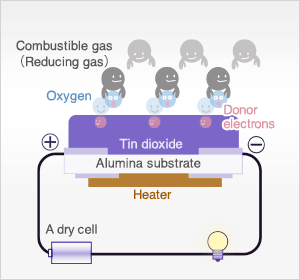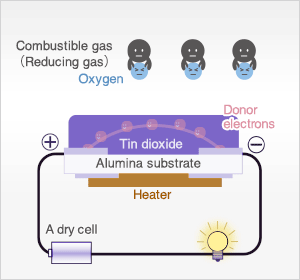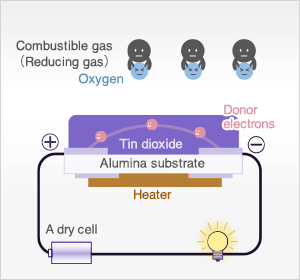
In clean air, donor electrons in tin dioxide are attracted toward oxygen which is adsorbed on the surface of the sensing material, preventing electric current flow.
STEP2
In the presence of reducing gases, the surface density of adsorbed oxygen decreases as it reacts with the reducing gases. Electrons are then released into the tin dioxide, allowing current to flow freely through the sensor.
Operating principle
When semiconductor particles (typically tin dioxide) are heated in air at high temperature, oxygen is adsorbed on the particle surface by capturing free electrons. The depletion layer thus formed is largely dependent on the radius of semiconductor particles used. If it is as small as conventionally used in gas sensors (tens nano-meters), the depletion can extend up to the whole area of each particle (volume depletion, high sensitive). If the size is far larger, on the other hand, depletion takes place conventionally on the periphery of each particle (regional depletion, low sensitive).
Figure 1 shows how the energy band structure and the distribution of conduction electrons change with increasing the partial pressure of oxygen from zero (flat band state) to state I (regional depletion), II (border) and III (volume depletion). Until the border is reached, the adsorption equilibrium is attained by increasing the depletion layer thickness. Later (volume depletion), however, the Fermi level is lowered by p kT on going from II to III while the layer thickness is kept constant.
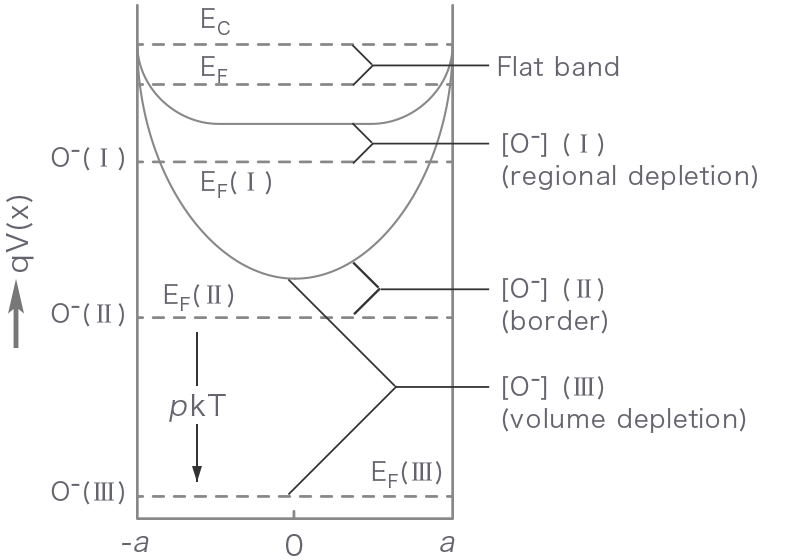
- x
- Distance in a radial direction
- qV(x)
- Potential energy
- a
- Particle radius
- [O-]
- Adsorbed oxygen concentration
- EC
- Conduction band energy
- EF
- Fermi level
- pkT
- Fermi level shift
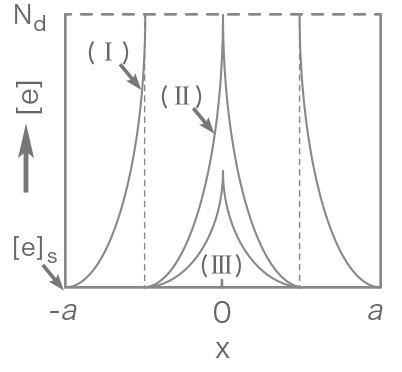
- [e]
- Electron concentration
- Nd
- Donor density
Figure 1. Energy band structure (top) and distribution of conduction electrons (bottom) for a semiconductor particle as correlated with an increase in adsorbed oxygen concentration
In this stage, two important equations are derived theoretically for a sensor device consisting of spherical particles, as follows.
Here [e]S is the surface electron concentration of particles and LD is the Debye length. R and R0 is the sensor resistances at the steady state and flat band state, respectively. For other symbols, see the caption of Fig.1. When sensor materials are selected, Nd, a, LD and R0 are fixed, while p is dependent on the actual gaseous conditions.
As described above, MOS type gas sensors change resistance (R) as a result of a change in adsorbed oxygen concentration. If this is used adequately, one can detect reducing gases like carbon monoxide. The adsorbed oxygen formed in clean air will be consumed on contact with carbon monoxide, the resulting decrease of R being used to estimate the concentration of carbon monoxide. The sensor recovers the original level of resistance when carbon monoxide is off. Such a detection mechanism is operative in tin dioxide based gas sensors.
| Reference: |
Noboru Yamazoe, Kengo Shimanoe, Basic approach to the transducer function of oxide semiconductor gas sensors, Sensors and Actuators B 160 (2011) 1352-1362 |
|---|

